Among the numerous phenomena in physics, the diffusion process is one of the simplest and most understandable. After all, every morning, preparing himself fragrant tea or coffee, a person has the opportunity to observe this reaction in practice. Let's learn more about this process and the conditions for its flow in different aggregate states.
What is diffusion
This word refers to the penetration of molecules or atoms of one substance between similar structural units of another. In this case, the concentration of penetrating compounds is leveled.
This process was first described in detail by the German scientist Adolf Fick in 1855
The name of this term was formed from the Latin verbal noun diffusio (interaction, dispersion, distribution).
Diffusion in a liquid
The process under consideration can occur with substances in all three states of aggregation: gaseous, liquid and solid. For practical examples of this, just look atkitchen.

Stove-boiled borscht is one of them. Under the influence of temperature, the molecules of glucosin betanin (a substance due to which the beets have such a rich scarlet color) evenly react with water molecules, giving it a unique burgundy hue. This case is an example of diffusion in liquids.
In addition to borscht, this process can also be seen in a glass of tea or coffee. Both of these drinks have such a uniform rich shade due to the fact that tea leaves or coffee particles, dissolving in water, evenly spread between its molecules, coloring it. The action of all popular instant drinks of the nineties is built on the same principle: Yupi, Invite, Zuko.
Interpenetration of gases
Continuing to look further for manifestations of the process in question in the kitchen, it is worth sniffing and enjoying the pleasant aroma emanating from a bouquet of fresh flowers on the dining table. Why is this happening?

Smell-carrying atoms and molecules are in active motion and, as a result, mix with particles already in the air, and are fairly evenly dispersed in the volume of the room.
This is a manifestation of diffusion in gases. It is worth noting that the very inhalation of air also belongs to the process under consideration, as well as the appetizing smell of freshly cooked borscht in the kitchen.
Diffusion in solids
The kitchen table with flowers is covered with a bright yellow tablecloth. She received a similar shade thanks tothe ability of diffusion to pass through solids.

The process of giving the canvas some uniform shade takes place in several stages as follows.
- Particles of yellow pigment diffused in the ink tank towards the fibrous material.
- They were then absorbed by the outer surface of the dyed fabric.
- The next step was to again diffuse the dye, but this time into the fibers of the fabric.
- In the final, the fabric fixed the pigment particles, thus becoming colored.
Diffusion of gases in metals
Usually, speaking about this process, consider the interaction of substances in the same state of aggregation. For example, diffusion in solids, solids. To prove this phenomenon, an experiment is carried out with two metal plates pressed against each other (gold and lead). The interpenetration of their molecules takes quite a long time (one millimeter in five years). This process is used to make unusual jewelry.

However, compounds in different aggregate states are also capable of diffusing. For example, there is diffusion of gases in solids.
During the experiments, it was proved that a similar process takes place in the atomic state. To activate it, as a rule, you need a significant increase in temperature and pressure.
An example of such gaseous diffusion in solids is hydrogen corrosion. It manifests itself in situations whereHydrogen atoms (Н2) that have arisen in the course of some chemical reaction under the influence of high temperatures (from 200 to 650 degrees Celsius) penetrate between the structural particles of the metal.
In addition to hydrogen, diffusion of oxygen and other gases can also occur in solids. This process, imperceptible to the eye, does a lot of harm, because metal structures can collapse because of it.
Diffusion of liquids in metals
However, not only gas molecules can penetrate into solids, but also liquids. As in the case of hydrogen, most often this process leads to corrosion (when it comes to metals).

A classic example of liquid diffusion in solids is the corrosion of metals under the influence of water (H2O) or electrolyte solutions. For most, this process is more familiar under the name of rusting. Unlike hydrogen corrosion, in practice it has to be encountered much more often.
Conditions for accelerating diffusion. Diffusion coefficient
Having de alt with the substances in which the process under consideration can occur, it is worth learning about the conditions for its occurrence.
First of all, the speed of diffusion depends on the state of aggregation of the interacting substances. The greater the density of the material in which the reaction occurs, the slower its rate.
In this regard, diffusion in liquids and gases will always be more active than in solids.
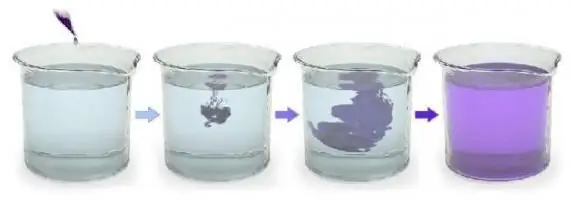
For example, if the crystalspotassium permanganate KMnO4 (potassium permanganate) throw into water, they will give it a beautiful raspberry color in a few minutes Colour. However, if you sprinkle crystals of KMnO4 on a piece of ice and put it all in the freezer, after a few hours, potassium permanganate will not be able to fully color the frozen H 2O.
From the previous example, one more conclusion about the diffusion conditions can be drawn. In addition to the state of aggregation, temperature also affects the rate of interpenetration of particles.
To consider the dependence of the process under consideration on it, it is worth learning about such a concept as the diffusion coefficient. This is the name of the quantitative characteristic of its speed.
In most formulas, it is denoted with a capital Latin letter D and in the SI system it is measured in square meters per second (m² / s), sometimes in centimeters per second (cm2 /m).
The diffusion coefficient is equal to the amount of matter dissipating through a unit surface over a unit of time, provided that the density difference on both surfaces (located at a distance equal to a unit length) is equal to one. The criteria that determine D are the properties of the substance in which the particle scattering process itself takes place, and their type.
The dependence of the coefficient on temperature can be described using the Arrhenius equation: D=D0exp(-E/TR).
In the considered formula E is the minimum energy required to activate the process; T - temperature (measured in Kelvin, not Celsius); R-gas constant characteristic of an ideal gas.
In addition to all of the above, the rate of diffusion in solids, liquids in gases is affected by pressure and radiation (inductive or high-frequency). In addition, much depends on the presence of a catalytic substance, often it acts as a trigger for the start of active particle dispersion.
Diffusion equation
This phenomenon is a particular form of a partial differential equation.
Its goal is to find the dependence of the concentration of a substance on the size and coordinates of the space (in which it diffuses), as well as time. In this case, the given coefficient characterizes the permeability of the medium for the reaction.

Most often, the diffusion equation is written as follows: ∂φ (r, t)/∂t=∇ x [D(φ, r) ∇ φ (r, t)].
In it φ (t and r) is the density of the scattering substance at point r at time t. D (φ, r) - generalized diffusion coefficient at the density φ at the point r.
∇ - vector differential operator whose components are partial derivatives in coordinates.
When the diffusion coefficient is density dependent, the equation is non-linear. When not - linear.
Having considered the definition of diffusion and the features of this process in different environments, it can be noted that it has both positive and negative sides.






