Ideal gas, the ideal gas equation of state, its temperature and pressure, volume… the list of parameters and definitions used in the corresponding section of physics can be continued for quite a long time. Today we will talk just on this topic.
What is considered in molecular physics?

The main object considered in this section is an ideal gas. The ideal gas equation of state was obtained taking into account normal environmental conditions, and we will talk about this a little later. Now let's approach this "problem" from afar.
Let's say we have some mass of gas. Its state can be determined using three parameters of a thermodynamic nature. These are, of course, pressure, volume and temperature. The equation of the state of the system in this case will be the formula for the relationship between the corresponding parameters. It looks like this: F (p, V, T)=0.
Here, for the first time, we are slowly approaching the emergence of such a thing as idealgas. It is called a gas in which the interactions between molecules are negligible. In general, this does not exist in nature. However, any highly rarefied gas is close to it. Nitrogen, oxygen and air, which are under normal conditions, are not much different from the ideal. To write the equation of state for an ideal gas, we can use the unified gas law. We get: pV/T=const.
Related Concept 1: Avogadro's Law
He can tell us that if we take the same number of moles of absolutely any random gas and put them in the same conditions, including temperature and pressure, then the gases will occupy the same volume. In particular, the experiment was carried out under normal conditions. This means that the temperature was 273.15 Kelvin, the pressure was one atmosphere (760 millimeters of mercury, or 101325 Pascals). With these parameters, the gas occupied a volume equal to 22.4 liters. Therefore, we can say that for one mole of any gas, the ratio of numerical parameters will be a constant value. That is why it was decided to designate this figure with the letter R and call it the universal gas constant. Thus, it equals 8.31. The unit is J/molK.
Ideal gas. The ideal gas equation of state and its manipulation
Let's try to rewrite the formula. To do this, we write it in this form: pV=RT. Next, we perform a simple action, multiply both sides of the equation by an arbitrary number of moles. We get pVu=uRT. Let us take into account the fact that the product of the molar volume andthe amount of matter is simply the volume. But after all, the number of moles will simultaneously be equal to the quotient of the mass and the molar mass. This is exactly what the Mendeleev-Clapeyron equation looks like. It gives a clear idea of what kind of system an ideal gas forms. The equation of state for an ideal gas will take the form: pV=mRT/M.
Deduce the formula for pressure
Let's do some more manipulations with the obtained expressions. To do this, the right side of the Mendeleev-Clapeyron equation is multiplied and divided by the Avogadro number. Now we carefully look at the product of the amount of substance by the Avogadro number. This is nothing but the total number of molecules in the gas. But at the same time, the ratio of the universal gas constant to the Avogadro number will be equal to the Boltzmann constant. Therefore, formulas for pressure can be written as follows: p=NkT/V or p=nkT. Here the symbol n is the particle concentration.
Ideal gas processes
In molecular physics there is such a thing as isoprocesses. These are thermodynamic processes that take place in the system at one of the constant parameters. In this case, the mass of the substance must also remain constant. Let's look at them more specifically. So, the laws of an ideal gas.
Pressure stays constant
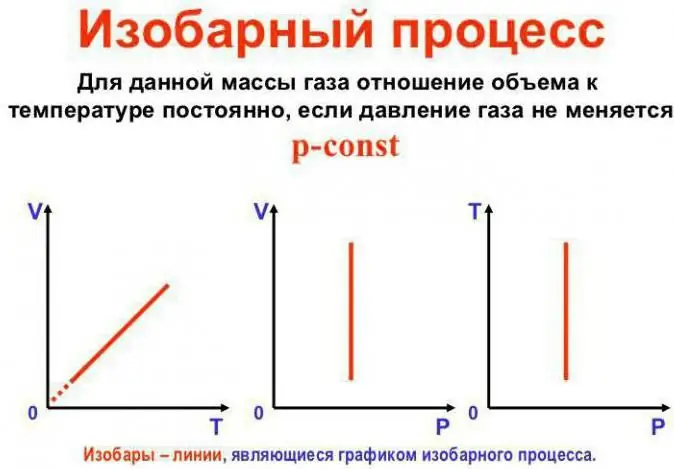
This is Gay-Lussac's law. It looks like this: V/T=const. It can be rewritten in another way: V=Vo (1 + at). Here a is equal to 1/273.15 K^-1 and is called the "volume expansion coefficient". We can substitute the temperature in both Celsius andthe Kelvin scale. In the latter case, we get the formula V=Voat.
Volume stays constant

This is Gay-Lussac's second law, more commonly referred to as Charles' law. It looks like this: p/T=const. There is another formulation: p=po (1 + at). Transformations can be carried out in accordance with the previous example. As you can see, the ideal gas laws are sometimes quite similar to each other.
Temperature stays constant

If the temperature of an ideal gas remains constant, then we can get the Boyle-Mariotte law. It can be written like this: pV=const.
Related Concept 2: Partial Pressure
Let's say we have a vessel with gases. It will be a mixture. The system is in a state of thermal equilibrium, and the gases themselves do not react with each other. Here N will denote the total number of molecules. N1, N2 and so on, respectively, the number of molecules in each of the components of the mixture. Let us take the pressure formula p=nkT=NkT/V. It can be opened for a specific case. For a two-component mixture, the formula will take the form: p=(N1 + N2) kT/V. But then it turns out that the total pressure will be summed from the partial pressures of each mixture. So, it will look like p1 + p2 and so on. These will be the partial pressures.
What is it for?
The formula we obtained indicates that the pressure in the system comes from each group of molecules. Incidentally, it does not depend onothers. D alton took advantage of this when formulating the law, later named after him: in a mixture where gases do not chemically react with each other, the total pressure will be equal to the sum of the partial pressures.






